Describe the Shape of a Water Molecule
Strong covalent bonds are formed between two or more water molecules. Water has 8 electrons around the central oxygen atom.

Bent Shape Of The Water Molecule H2o Youtube
The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.

. Remaining two hybrid orbitals are occupied by two lone pairs. Describe the shape of a water molecule Bent into a V shape. A water molecule consists of two atoms of hydrogen linked by covalent bonds to the same atom of oxygen.
The molecule of water. For example molecules CO2 and H 2O both have three atoms. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties and there are few molecules that are more stable and difficult to decompose than H 2 O.
Select all that apply. The geometry of molecules depends on the number of atoms present in the molecule and the angles between bonds in the molecule. Consequently the electrons in the water molecule spend slightly more time around the oxygen atomic center and less time around the hydrogen atomic centers.
2 hydrogens and 1 oxygen asymmetrical distribution of charges dipolar structure. There are two bonding pairs and two lone pairs. Your explanation should take into account.
So water molecule has four hybrid orbitals. Walter the Water Molecule Take a Journey Through the Water Cycle nearby. In a water molecule each hydrogen atom shares an electron pair with the oxygen atom.
The geometry of the water molecule is dictated by the shapes of the outer electron orbitals of the oxygen atom which are similar to the bonding orbitals of carbon. Provide an explanation as to why water. These orbitals describe a rough tetrahedron with a hydrogen atom.
Polarity of the water molecule Owing to the electronegativity difference between hydrogen H and oxygen O atoms and the bent shape of the H 2 O molecule a net dipole moment exists. Water molecules are polar and thus associate with each other through hydrogen bonds. Water molecule is polar because of difference in eletronegativities of O and H.
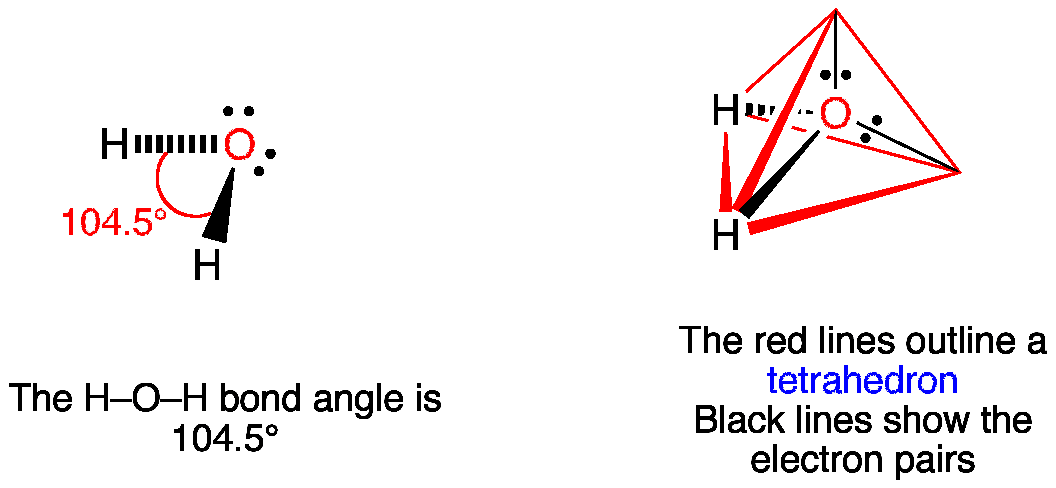
Water is a molecular compound consisting of polar molecules that have a bent shape. Draw the structure of a water molecule and describe its shape and using VSEPR. The angle between bonds in water is 1045 making water a bent molecule.
When solutes are added to water they may. The oxygen atom in a water molecule is strongly electronegative. The angle between bonds is 180 in carbon dioxide making this molecule linear.
In water each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them. Water H2O is also a compound. Which options describe the structure of a water molecule.
However the electrons of the covalent bonds are not shared equally between the oxygen and hydrogen. The atoms of the water molecule are held together by covalent bonds. The oxygen atom acquires a partial negative charge while the hydrogen atom acquires a partial positive charge.
It has become a cloud. The bond between hydrogen and oxygen is 105 which is slightly less than the idealized 1095 degree angle of sp3 hybridized orbitals. Water H 2 O is polar because of the bent shape of the molecule.
Other water molecules are doing the same thing nearby forming tiny drops of water around the dust particles. H 2 O that contains two hydrogen atoms each sharing a pair of electrons with an oxygen atom see Figure 1. Polarity of a Water Molecule.
The hydrogen atoms are attached at opposite ends of the oxygen atom. Why Is Water H2o A Polar Molecule Youtube The Structure Of Water Chemistry For Non Majors. A water molecule has a single Oxygen atom bound to two Hydrogenatoms at approximately 120o.
Atoms of oxygen are electronegative and attract the shared electrons in their covalent bonds. When atoms share electrons in this way a covalent bond A chemical bond created by the sharing of electrons between atoms. Water has nonpolar bonds but it has a bent shape and is therefore polar.
The hydrogen atoms are attached to the oxygen atom at an angle 3. This means there are four electron pairs arranged in a tetrahedral shape. This is an example of polar covalent chemical bonding.
Covalent bonds in a water molecule bind its oxygen atom to its two hydrogen atoms. Molecules are considered to be polar. Water is a molecule A combination of two or more atoms bound together that has different qualities than the individual atoms.
Its structure consists of two hydrogen atoms joined to one oxygen atom by single covalent bonds. The molecule has an overall straight shape 2. A water molecule is polar but it has no net charge.
The resulting shape is bent with an H-O-H angle of 1045. Structure of Water molecule. The molecule has an overall bent shape 4.
Think of Mickey Mouses head - hisface would be the Oxygen atom and his ears would be. Because of lone pair-lone pair repulsion shape of becomes bent. Water is considered to be a polar molecule.
Theory provide an explanation for the shape of the molecule. The figure indicates the partial charges that the atoms possess. The unequal sharing of negatively charged electrons combined with its V shape makes a water molecule polar.
Water molecules have a bent structure. Looking down Walter sees that the crowd of tiny water drops around him is casting a shadow on the treetops below. However if you have to describe the ion you can use the phrase the like a polar molecule because I3- is soluble in water.
Water has polar bonds and a linear symmetrical shape so it is polar. Why are the bonds in a water molecule polar covalent and how does that affect the interactions between water molecules. Water molecules have slight charges and are attracted to each other by ionic bonds.
Two hybrid orbitals form 2 sigma bond with two H atoms.

Molecular Shapes Chemistry For Non Majors
Comments
Post a Comment